BTNX in Hot Water After Deletion of Crucial Data

In a surprising turn of events, a small Toronto-based rapid test importer, BTNX, secured an estimated $2 billion in federal contracts during 2021 and 2022, despite a year-long investigation revealing concerning practices. Global News discovered that BTNX deleted crucial data from a study submitted to Health Canada, presenting its COVID-19 test as more reliable than it actually was.
The investigation disclosed that the deleted specimens skewed the test's accuracy, making it more likely to produce false-negative results, potentially jeopardizing Canadian lives. Jillian Kohler, director of the University of Toronto’s World Health Organization Collaborating Centre, expressed outrage over the discrepancies in testing that had real-life implications.
BTNX, initially a harm reduction kit supplier, became the leading rapid test supplier in Canada during the pandemic. Despite lacking specific expertise in infectious diseases, the company secured 15 contracts, becoming a crucial part of the nation's pandemic response. The tests, imported from China, became widely used for various purposes, from workplace screenings to visiting loved ones in long-term care facilities.
The investigation uncovered that BTNX sourced its tests from Assure Tech in China and deleted data from a study, raising ethical concerns among researchers. While BTNX maintains its transparency, experts criticize the apparent flaws in the test's reliability.
The controversy raises questions about the Canadian government's decision-making, as Health Canada and the Public Health Agency purchased 404 million tests from BTNX. Despite concerns, Health Canada defended the tests' scientific integrity, emphasizing the importance of following fine-print instructions and verifying results with PCR tests.
As Canadians face a potential uptick in COVID-19 infections during the holidays, the controversy surrounding BTNX's tests adds uncertainty. With BTNX threatening legal action and Health Canada showing no immediate plans for investigation, the aftermath of this revelation leaves many with lingering questions and concerns.
READ THE FULL GLOBAL NEWS ARTICLE HERE
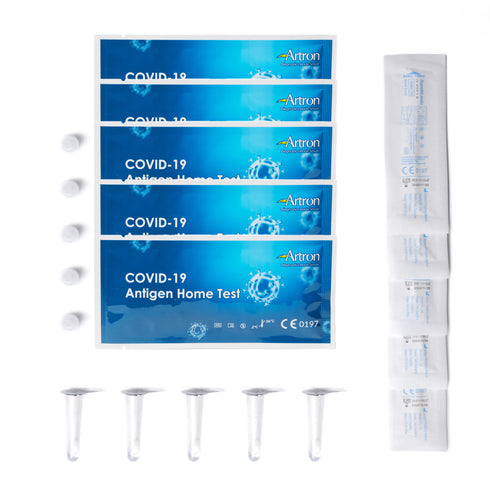
Upon learning this news, the team here at PPE Supply Canada took swift action and has since removed all BTNX rapid test kits from our inventory. We work hard to ensure all of our customers are equipped with reliable PPE, which is why we have introduced the Artron COVID-19 Rapid Test Kit to our catalogue! Made right here in Canada, Artron gives you results in just 15 minutes! Use the Artron Rapid Response COVID-19 Antigen Test to conveniently check yourself when symptoms emerge, just after a crowded event, or returning from a trip! Easy to use and packaged in a convenient box of 5, these Health-Canada-approved rapid test kits are a great tool to have on hand whether at home or work! Keep yourself, your household, or your team safe and test regularly!
Opting for Artron Rapid Test Kits means backing your fellow Canadians, as every Artron Rapid Response COVID-19 Antigen Test Kit is proudly manufactured in Canada!
Shop local, shop Canadian!
SHOP THE ARTRON RAPID COVID-19 TEST KIT HERE
The Artron Rapid Antigen COVID test is authorized by Health Canada (Interim Authorization Number: 350132)